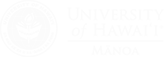
We have ongoing work in three areas: total synthesis, the development of new synthetic methods and in medicinal chemistry. A long-term collaborative effort with the medicinal chemistry group of Professor Alexandros Makriyannis (Center for Drug Discovery, Northeastern University) involves the preparation of ligand for mapping the CB1 and CB2 receptors. More recently our group has been collaborating with Dr. James Turkson (Cedars-Sinai Medical Center, Los Angeles) on the design and synthesis of small molecule inhibitors of STAT3, a protein involved in cell signaling.
Our group’s approach to total synthesis is largely driven by a desire to apply the new methods we develop to natural products synthesis. Our overarching goal is to design brief syntheses that can be implemented by a small team. We recently disclosed the synthesis of both enantiomers of rocaglamide in which an asymmetric Pd(0)-catalyzed Nazarov-like cyclization developed in our group was the key step. We are currently working on a second-generation synthesis of rocaglamide by means of a different strategy.
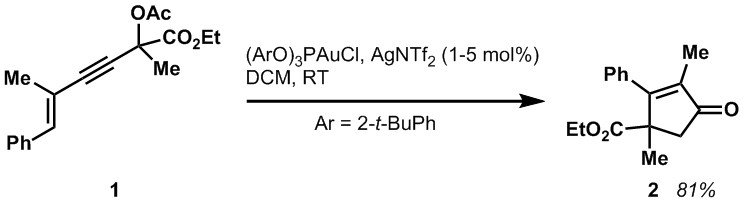
Our most recent methods development projects have focused both on racemic and catalytic asymmetric versions of the Nazarov cyclization. In the past we had developed a series of very effective pyranose-derived chiral auxiliaries for use in the allene ether version of the Nazarov cyclization. More recently, we reported a cationic Au(I) catalyzed Nazarov cyclization of alkyne 1 that led to cyclopentenones, such as 2, incorporating vicinal all-carbon atom quaternary stereocenters. We plan to explore an asymmetric version of this reaction.
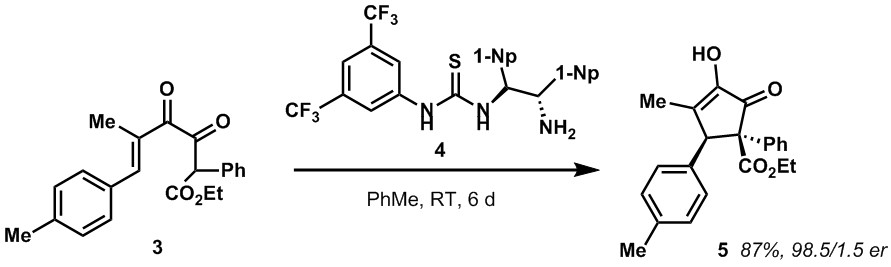
We have been fairly successful in our past efforts to develop asymmetric versions of the Nazarov cyclization. For example, we developed a thiourea based organocatalyst 4 that is able to effect high levels of enantioselectivity for the cyclization of alpha-diketoenones. Since then we have directed our attention to the development of new organocatalysts as well as transition metal based catalysts for the Nazarov cyclization.
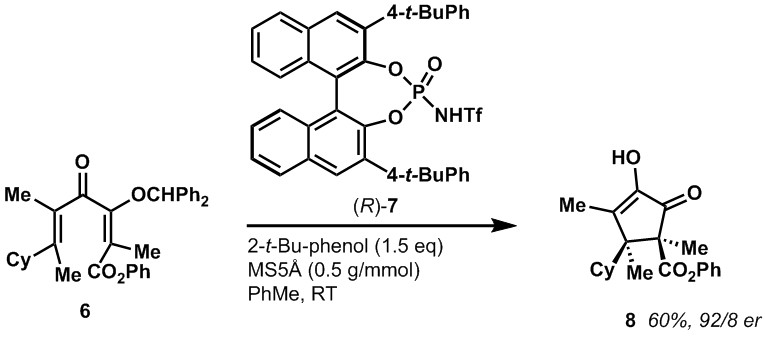
Our group developed a chiral Bronsted acid organocatalyst 7 that cyclized dienones, such as 6, with high levels of enantioselectivity. These cyclopentenones represent a new solution to a long withstanding problem in organic synthesis – the controlled asymmetric synthesis of vicinal quaternary all-carbon stereocenters. It is noteworthy that the catalyst was able to mediate the cyclization of unactivated, all-aliphatic dienones with good enantioselectivities. Most related work only functions for substrates bearing at least one aromatic ring. The success of this Nazarov cyclization was in part dependent on being able to prepare geometrically pure fully substituted dienones, which itself is a synthetic challenge.
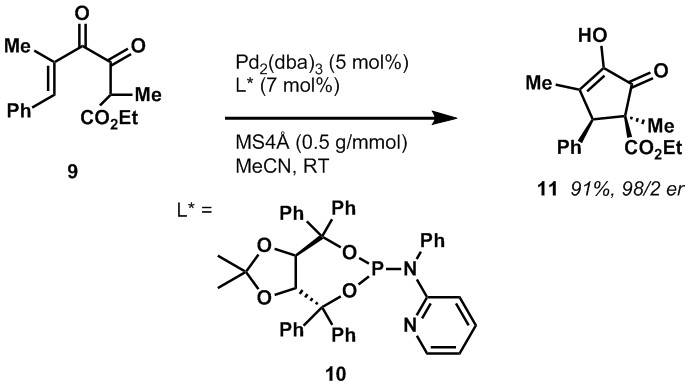
After our group’s initial discovery of a Pd(0)-catalyzed Nazarov-like cyclization of alpha-diketoenones, we were interested in developing an enantioselective version of this reaction. In the course of this work we developed a new TADDOL-derived phosphoramidite ligand 10 for Pd(0) which is capable of inducing asymmetry at the alpha-diketoenone to produce cyclopentenones with a high degree of enantioselectivity.
We work on other methods in addition to the Nazarov cyclization. These either suggest themselves over time or were accidentally discovered in the pursuit of other goals. We have two such methods in development at present. The first involves an oxidative cascade process to produce non-planar polynuclear aromatic compounds and the other is a stereoselective synthesis of (Z)-trisubstituted trifluoromethyl alkenes.