-
Distilled water
-
Ethyl alcohol
-
Vegetable oil
-
Sodium chloride (table salt)
-
Sugar
-
Starch
-
Baking soda
-
10 mL graduated cylinder
-
Stopper to fit graduated cylinder
-
Containers and lids to store solutions
-
Labeling tape
-
Waterproof marking pen
|
Liquid |
Bonding |
Polarity |
Model Molecule |
|
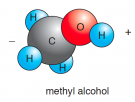
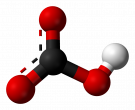
Distilled water* |
Covalent |
Highly polar |
|
|
Alcohol |
Covalent |
Slightly polar |
|
|
Oil ** |
Covalent |
Nonpolar |
|
- Use the information on the liquids in Table 3.3 and solids in Table 3.4 to predict how much of each solid solute the solvent will dissolve.
- Determine how much solid solute each solvent can dissolve.
- Obtain the solvents and solutes you are assigned to work with.
- Test the solubility of the solutes in the solvent. Put 2 mL of your first solute into a 10 mL graduated cylinder, then fill the graduated cylinder to the 10 mL line with your solvent liquid.
- Stopper the cylinder, shake the contents gently for 10 seconds, and let the mixture stand until settling stops, approximately 20 seconds. Shaking vigorously may temporarily form a suspension.
- Determine how much of the solid dissolved. Record your observations as all, some, or none in Table 3.4.
- Optional: Save your solution to test conductivity. Store in containers with airtight lids to prevent evaporation. Label the containers with your name and the solution.
- Wash the graduated cylinder and stopper and rinse with tap water.
- Repeat steps (b) to (g) for each of the other solids shown in Table 3.5
- For your assigned solvent, predict how much of each of the other liquids it can dissolve. Using the terms all, some, and none, record your predictions in Table 3.6.
- Determine the capacity of each liquid solvent to dissolve other liquids.
- Put 2 mL of test liquid solute into a 10 mL graduated cylinder; then fill the graduated cylinder to the 10 mL line with your solvent liquid.
- Stopper the cylinder, shake the contents gently for 10 seconds, and let the mixture stand till settling stops.
- Observe how the solvent liquid dissolved in the test liquid. Determine how much of the test liquid dissolved. Record your observations as all, some, or none.
- If there is a separation of the liquids, measure the volume of the bottom layer. Record the volume and identity of the bottom layer in Table 3.6.
- Wash the graduated cylinder and stopper and rinse with tap water.
- Repeat steps (a) to (e) for each of the other solvents shown in Table 3.6
|
Solute |
Chemical Formula |
Bonding |
Polarity / Charged Ions |
Model Molecule |
|
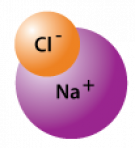
Salt |
NaCl |
Ionic |
Highly polar |
|
|
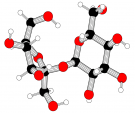
Table Sugar (sucrose) |
C12H22O11 |
Covalent (one glucose sugar and one fructose sugar bonded together) |
Polar (due to negatively charged OH- groups) |
Image from Wikipedia Commons |
|
Starch |
(C6H10O5)n n is usually a large number |
Covalent (long chains of the sugar glucose) |
Nonpolar (due to branching of molecule) |
|
|
Baking soda |
NaHCO3 |
Ionic (compound of a covalent polyatomic ion HCO3- and Na+)
|
Charged ions |
|
- How do you know when:
- a solid has completely dissolved in a liquid?
- a liquid has completely dissolved in another liquid?
- What statements can be made about the solubility of polar, nonpolar, and ionic solutes in
- nonpolar liquid solvents
- slightly polar liquid solvents
- ionic solvents
- Knowing that water is polar, what generalization can you make about powdered drink mix?
- Minerals dissolve and are carried from the land in stream water. Soil is also carried in stream water, but does not dissolve. What can you infer about minerals and soil?