Water has an amazing ability to adhere (stick) to itself and to other substances.
Hydrogen Bonds
Hydrogen bonds form when hydrogen atoms covalently bonded to nitrogen (N), oxygen (O), or fluorine (F) in the form of covalent compounds such as ammonia (NH3), water (H2O) and hydrogen fluoride gas (HF). In these molecules, the hydrogen atoms do not pull as strongly on the shared electrons as the N, O, or F atoms. Therefore, the molecules are polar; the hydrogen atoms become positively charged and are able to form hydrogen bonds to negative ions or negatively charged parts of other molecules (such as the N, O, and F atoms that become negatively charged in these compounds).
Hydrogen bonds are not true bonds like covalent bonds or ionic bonds. Hydrogen bonds are attractions of electrostatic force caused by the difference in charge between slightly positive hydrogen ions and other, slightly negative ions. These attractions are much weaker than true ionic or covalent bonds, but they are strong enough to result in some interesting properties.
Fig. 3-7: Hydrogen bonds shown as the dotted lines between water molecules.
In the case of water, hydrogen bonds form between neighboring hydrogen and oxygen atoms of adjacent water molecules. The attraction between individual water molecules creates a bond known as a hydrogen bond. See Fig. 3-7.
A molecule of water has two hydrogen atoms. Both of these atoms can form a hydrogen bond with oxygen atoms of different water molecules. Every water molecule can be hydrogen bonded with up to three other water molecules (See Fig. 3-7). However, because hydrogen bonds are weaker than covalent bonds, in liquid water they form, break, and reform easily. Thus, the exact number of hydrogen bonds formed per molecule varies.
Cohesion
Molecules of pure substances are attracted to themselves. This sticking together of like substances is called cohesion. Depending on how attracted molecules of the same substance are to one another, the substance will be more or less cohesive. Hydrogen bonds cause water to be exceptionally attracted to each other. Therefore, water is very cohesive.
We see evidence of water’s cohesiveness every day – in water drops and in streams of water. Our experience with water, however usually involves water touching something else or being acted upon by gravity. To really get a sense of water’s cohesiveness, scientists looked at the behavior of water in space (see Fig. 3-8). In space, water is able to form perfectly round spheres because the attraction of water to itself pulls the water into the shape with the least amount of surface area compared to the volume – a sphere.
Fig. 3-8: Water drops in space. (A)European Space Agency astronaut Pedro Duque of Spain watches a water bubble float between him and the camera, showing his image refracted, on the International Space Station. (B) A large water sphere made on a 5 cm diameter wire loop by U.S. astronaut Dr. Pettit.
Adhesion
Adhesion is similar to cohesion, but it involves unlike (i.e. different) substances sticking together. Water is very adhesive; it sticks well to a variety of different substances. Water sticks to other things for the same reason it sticks to itself – because it is polar so it is attracted to substances that have charges.
Water adheres to many things— it sticks to plants, it sticks to dishes, and it sticks to your eyebrows when you sweat. In each of these cases water adheres to or wets something because of adhesion. This is why your hair stays wet after you shower. Molecules of water are actually sticking to your hair (Fig. 3-9). Adhesion also explains why soil is able to hold water (and form mud).
Photo courtesy of Mo Riza
Photo by Alyssa Gundersen
Fig. 3-9: Child with wet hair (a) and enlarged photo of individual drops of water on wet hair (b).
Activity
Investigate the cohesive and adhesive properties of water.
Surface Tension
Fig. 3-11: Water piled on top of a penny showing surface tension caused by the cohesive property of water and hydrogen bonding
The cohesion of water creates surface tension where air and water meet. You observed this in Activity 2 when you looked at the ability of water to pile on top of a penny without spilling over (see Fig. 3-11).
Fig. 3-12: Picture of red rover game.
Photo courtesy of Michael Sarver
The hydrogen bonds between water molecules at the surface are analogous to the to members of a red rover team holding hands. When playing red rover, team members line up to form a chain to try and prevent someone from running through their joined hands (Fig. 3-12). The linked hands represent the hydrogen bonds between water molecules that can prevent an object from breaking through.
Of course, a faster or heavier person can more easily break through the hand bonds during a game of red rover. Similarly a heavy object, or one that isn’t carefully placed on the surface of the water, can break the surface tension. Remember, for example, how the paper clip needed to be placed carefully on the water’s surface in order for it to float (Activity 2).
Fig. 3-13: Water molecules at the surface of a liquid demonstrating surface tension.
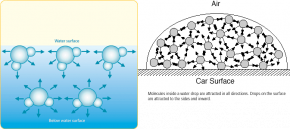
Where air and liquids meet there are unbalanced forces. Water molecules very near the surface are being pulled down and to the side by the strong cohesion of water to itself and the strong adhesion of water to the surface it is touching. In contrast, the air pulling upward acts as an extremely small force on the water’s surface. The result is a net force of attraction between water molecules a very flat, thin sheet of molecules at the surface (see Fig. 3-13).
Because of hydrogen bonding, water can actually support objects that are more dense than it is. Water molecules stick to one another on the surface, which prevents the objects resting on the surface from sinking. This is why water striders and other insects can “walk” on water! It is also what allowed you to float a paper clip on water and the reason why a belly flop off the high dive into a pool of water is painful. See Fig. 3-14.
Image copyright and source
Photo courtesy of Alexander Yurusov
Image caption
B. A paperclip floating on water
Image copyright and source
Image copyright and source
Photo courtesy of Sarah Martin
Fig. 3-15: Rulers stuck together.
In Activity 2, you tried to stick two rulers together using a thin film of water between the rulers. Water acted like glue, and you were able to use one ruler to lift the other ruler using the adhesiveness of water (see Fig. 3-15). This was a result of both water-water cohesion and water-ruler adhesion.
In fact, because liquid water is so good at sticking to itself and to other substances, it can rise up a surface against the force of gravity! We call this climbing tendency of water capillarity (also called capillary action). You saw capillarity in Activity 2 when you placed glass tubing in water.
Capillarity starts when the water molecules nearest the wall of the tube are attracted to the tube more strongly than to other water molecules. The water molecules nearest the glass wall of the tube rise up the side (adhesion), dragging other water molecules with them (cohesion). Water level in the tube rises until the downward force of gravity becomes equal to than the adhesion and cohesion of water.
Fig. 3-16: Capillarity in different sized glass tubes.
In a narrow tube, the molecules at the edges have fewer other water molecules to drag up the tube than in a large tube. Therefore, water can rise higher in a narrow tube than in a wider tube (see Fig. 3-16). Capillarity happens naturally in soils, fabric, and wherever there are small spaces that liquids can move through.