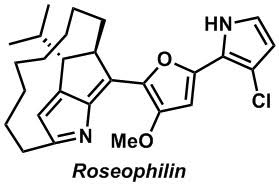
One of our long standing goals is to apply the methods that we develop to the synthesis of natural products. Our benchmark is to design and implement total syntheses of no more than 15 linear steps. We place this somewhat arbitrary restriction on ourselves because these syntheses can be accomplished with a small team without having them evolve into problems of logistics. Not too long ago we developed the synthesis of both enantiomers of rocaglamide through a synthesis that made use of the Pd(0) catalyzed Nazarov-like cyclization that we had discovered. We are currently working on a second-generation approach that makes use of some of the same starting materials that we developed for our first synthesis, but employs completely different logic. Ideally, the molecules that we choose to prepare should have some interest beyond chemistry. In the case of rocaglamide, the interest derives from the potent cytostatic and anti-inflammatory activity.
Recent Publications
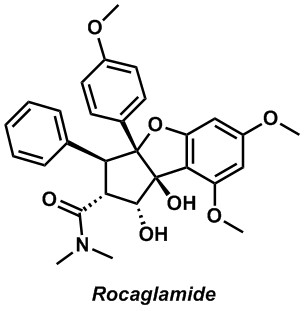
Rocaglamide
Zhou, Z.; Dixon, D. D.; Jolit, A.; Tius, M. A., The Evolution of the Total Synthesis of Rocaglamide. Chem. Eur. J. 2016, 22, 15929-15936
DOI: 10.1002/chem.201603312
AND
Zhou, Z.; Tius, M. A., Synthesis of Each Enantiomer of Rocaglamide by Means of a Palladium(0)-Catalyzed Nazarov-Type Cyclization. Angew. Chem. Int. Ed., 2015, 54, 6037-6040.
DOI: 10.1002/anie.201501374

Terpestacin
Berger, G. O; Tius, M. A., Total Synthesis of (±)-Terpestacin and (±)-11-epi-Terpestacin. J. Org. Chem. 2007, 72, 6473-6480.
DOI: 10.1021/jo070923d
Madindoline A and B
Wan, L.; Tius, M. A., Synthesis of (+)-Madindoline A and (+)-Madindoline B. Org. Lett. 2007, 9, 647-650.
DOI: 10.1021/ol062919e