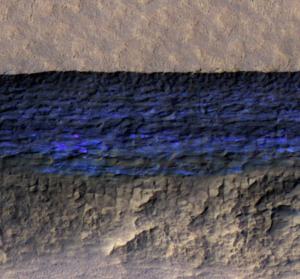
Could new theory answer what causes landslides on Mars?
University of Hawaiʻi at MānoaProfessor, HIGP, School of Ocean and Earth Science and Technology
Marcie Grabowski, (808) 956-3151
Outreach specialist, School of Ocean and Earth Science and Technology
Using Mars orbiter data, field observations and laboratory experiments, a team of researchers, including Peter Englert, professor in the University of Hawaiʻi at Mānoa School of Ocean and Earth Science and Technology (SOEST), developed a new theory about what is causing landslides on the surface of Mars. Their research was published in Science Advances.
Previous ideas suggested that liquid debris flows or dry granular flows caused this movement. However, neither model can completely account for the seasonal martian flow features known as Recurring Slope Lineae (RSL).
The team, led by Janice Bishop, SETI Institute senior research scientist and member of the NASA Astrobiology Institute team, alternatively hypothesizes that small-scale ice melting underground causes changes that make the surface vulnerable to dust storms and wind. As a result, the features appear or expand on the surface of Mars. Further, the team believes that the thin layers of melting ice result from interactions between underground water ice, chlorine salts and sulfates, which create an unstable, liquid-like flowing slush that instigates sinkholes, ground collapse, surface flows and upheave.
Earth sediments parallel Mars samples
Mars analog field investigations on Earth, such as in the Dry Valleys of Antarctica, the Dead Sea in Israel and Salar de Pajonales in the Atacama Desert, show that when salts including gypsum interact with water underground, it causes disruptions on the surface, including collapse and landslides.
The current project arose out of work on sediments from the McMurdo Dry Valleys in Antarctica, one of Earth’s coldest and driest regions. As on Mars, the Dry Valleys' surface is cold and scoured by dry winds most of the year. However, subsurface permafrost contains water ice, and chemical alteration appears to be occurring below the surface.
To test its theory, the team conducted lab experiments to observe what would occur if they froze and thawed analog samples comprised of chlorine salts and sulfates at low temperatures such as what would be found on Mars. The result was slushy ice formation near -50 °C, gradual melting of the ice from -40 to -20 °C and thin layers of liquid-like water forming along grain surfaces.
Englert, who is based at the Hawaiʻi Institute of Geophysics and Planetology in SOEST, managed efforts to analyze the chemistry of Antarctic sediments from several soil pits and cores, enhancing understanding of the salt enrichment in the near-surface layers.
“Because Dry Valley sediments are analogous to Mars sediments, our experiments can provide clues about the processes that may be occurring on Mars,” said Englert. “Elevated concentrations of chlorine salts and sulfates were found just below the surface in multiple locations we studied in Antarctica's Wright Valley. The ubiquitous subsurface presence of these salts in Antarctica suggest their presence on Mars and their potential role in triggering landslide processes.”
Dynamic martian environment
Modeling the behavior of chlorine salts and sulfates, including gypsum, under low temperatures demonstrated how interrelated these salts are. It may be that this microscale liquid water migrates underground on Mars, transferring water molecules between the sulfates and chlorides, almost like passing a soccer ball down the field.
“I was thrilled to observe such rapid reactions of water with sulfate and chlorine salts in our lab experiments and the resulting collapse and upheave of Mars analog soil on a small scale, replicating geologic collapse and upheave features in karst systems, salt reservoirs and edifice collapse on a large scale,” said Bishop.
“I am excited about the prospect of microscale liquid water on Mars in near-surface environments where ice and salts are mixed with the soil,” added Bishop. “This could revolutionize our perspective on active chemistry just below the surface on Mars today.”