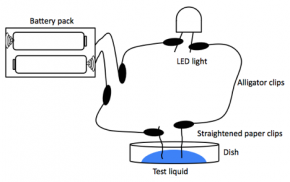
Fig. 3.23. Conductivity-testing diagram
Title
Activity: Conductivity
Table of Contents
A simple conductivity meter can be made with a light bulb and a battery. Use a simple conductivity meter to compare the conductivity of polar, slightly polar and nonpolar liquids.
Materials
- Two straightened paper clips
- Battery pack (two AA batteries in holder)
- LED light with resistor
- Three alligator clips
- Six containers large enough to hold 10 ml of liquid (e.g. petri dish)
- Small cup
- Distilled water
- Tap water
- Salt water
- Vegetable oil
- Piece of dark colored construction paper or a dark counter top
- Paper towel
- Solutions saved from Solubility Activity (optional)
Procedure
- Investigate the electrical properties of your LED light.
- Using only the battery and the LED determine how to light the LED.
- Record you observations of how orientation affects your ability to light the LED and any other procedures required to generate light.
- Invent and record a hypothesis for your observations.
- Compare your LED to that of your classmates.
- Record observations of color and brightness.
- Invent and record a hypothesis for your observations.
- Determine if you can manipulate the battery pack and LEDs to:
- Light more than one LED at a time?
- Create a brighter LED?
- Record your observations and procedures that allow you to accomplish these manipulations.
- Invent and record a hypothesis for your observations.
- Using only the battery and the LED determine how to light the LED.
- Expand your LED and battery circuit to create a conductivity meter.
- Use the alligator clips to expand the circuit.
- Use the paper clips as the final electrodes that will be put into solution.
- Test your circuit by touching the two paper clip electrodes together to light the LED. If your LED does not light, manipulate the circuit until it does light. If you are having difficulty, refer to Fig. 3.23.
- Draw your circuit set-up.
- Investigate the conductivity of distilled water (pure water), tap water and salt water. You can measure the relative conductivity of liquid by observing the brightness of the LED, when the electrodes are placed in a solution that conducts electricity.
- Obtain 10 mL test solutions of distilled water, tap water, and salt water in Petri dishes. Fill a small cup with extra tap water. Use this tap water and a paper towel for cleaning electrodes between tests.
- Predict the conductivity of distilled water, tap water, and salt water in terms of their ability to light the LED. Use the following scale to translate your predicted conductivity estimates into predicted observations of the LED:
- Nonconductor = No light
- Poor conductor = Dim light
- Good conductor Bright light
- Use Table 3-7 to record your predictions.
- Test the conductivity of pure water, tap water, and salt water by placing the electrodes in each respective solution and observing the brightness of the LED. Your electrodes can be close but they cannot touch. Between observations, rinse your electrodes by dipping them into your container of extra tap water and wiping them on a paper towel after each rinse.
- Verify your observations by repeating the tests until you are confident in your results. You may need to place your LED on a dark background to more accurately observe its brightness.
- Use Table 3.7 to record your results. Remember that you are using your observation of LED brightness to make an inference about the conductivity of each solution.
- No light = Nonconductor
- Dim light = Poor conductor
- Bright light = Good conductor
- Expand your investigation of conductivity to oil.
- Use your knowledge of this covalent molecule to make a prediction about its conductivity and ability to light the LED using the same scale that you used for the different water solutions.
- Use Table 3-7 to record your predictions.
- Test the conductivity of oil by placing the electrodes in oil, as you did with water, and observing the brightness of the LED. Verify your observations by repeating the test until you are confident of your results.
- Use Table 3-7 to record your results.
- Optional: Further expand your investigation to test the conductivity of other liquids.
- Choose at least two other liquids to investigate. Use your prior knowledge about these liquids to make predictions about their conductivity. Use the same scale, based on LED brightness, that you used for the different water solutions.
- Use Table 3-7 to record your predictions.
- Test the conductivity of each solution by observing the brightness of the LED. Verify your observations by repeating the tests until you are confident of your results.
- Use Table 3-7 to record your results.
-
Optional: Further expand your investigation to test the conductivity of solutions of solids dissolved in various liquids from the Solubility Activity.
-
Transfer the observed amount of solute dissolved (from Table 3-4 in the Solubility Activity) to the column in Table 3-8 on the relative amount of solute dissolved in each liquid.
-
Use your knowledge of the dissolved solids (solutes, salt, sugar, and starch), and the solvents (distilled water, alcohol, and oil) to make predictions about their conductivity. Use the same scale, based on LED brightness, that you used for the different water solutions.
-
Use Table 3-8 to record your predictions.
-
Test the conductivity of each solution by observing the brightness of the LED. Verify your observations by repeating the tests until you are confident of your results.
-
Use Table 3-8 to record your results.
-
Activity Questions
- The LED lights need to be in a specific orientation in order to light up when connected to the battery pack. Describe the orientation and explain how you determined this.
- The conductivity of seawater is about one million times higher than distilled water. Did your results support this? Explain.
- Which result surprised you the most? Explain why it surprised you and then invent and describe a hypothesis to explain the results you observed.
- What did the conducting solutions have in common? Make a generalization about the types of materials that conduct electricity and the types of bonds those materials have.
- Would you rather be swimming in a lake or the ocean during a lightning storm? Why?