Covalent Compounds
Covalent Bonding
Ionic compounds, such as sodium chloride (NaCl), are formed by a transfer of electrons that creates ions. Ions exert electrostatic force on each other, which forms ionic bonds. The hydrogen and oxygen atoms in a water molecule, however, are bonded by sharing electrons rather than by transferring them.
Fig. 2.27. Puppies demonstrating covalent bonding (A) The two puppies represent atoms, their bones represent one of their electrons. (B) Both puppies share both bones.
Image by Byron Inouye
Imagine two puppies, each with a bone (Fig. 2.27 A). The puppies represent atoms. The bones represent one of their electrons. Both puppies share both bones (Fig. 2.27 B). This is how hydrogen and oxygen share electrons; they each have an electron that they can share in a bond. This is a covalent bond, a bond in which atoms share electrons. Covalent bonding generally happens between nonmetals. Covalent bonding is the type of bond that holds together the atoms within a polyatomic ion.
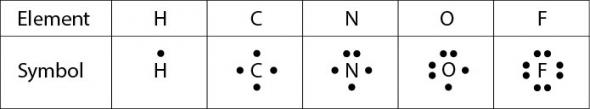
It takes two electrons to make a covalent bond, one from each bonding atom. Lewis dot structures are one way to represent how atoms form covalent bonds. A table of Lewis dot symbols of nonmetal elements that form covalent bonds is shown in Fig. 2.28 Dots are placed around the symbol of the element to represent the number of valence electrons in the element. There can be up to eight dots, for eight valence electrons. The first four electrons are placed as single electrons, then the remaining four are paired.
Fig. 2.28. Lewis dot symbols of nonmetal elements
Image by Byron Inouye
The number of bonds that each element is able to form is usually equal to the number of unpaired electrons. In order to form a covalent bond, each element has to share one unpaired electron.
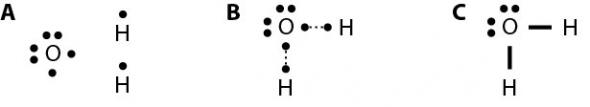
Fig. 2.29 gives an example of how to make a Lewis dot structure. First, determine how many atoms of each element are needed to satisfy the octet rule for each atom. In the formation of water, an oxygen atom has two unpaired electrons, and each hydrogen atom has one (Fig. 2.29 A). To fill its valence shell, oxygen needs two additional electrons, and hydrogen needs one. One oxygen atom can share its unpaired electrons with two hydrogen atoms, each of which need only one additional electron. The single electrons match up to make pairs (Fig. 2.29 B). The oxygen atom forms two bonds, one with each of two hydrogen atoms; therefore, the formula for water is H2O. When an electron, or dot, from one element is paired with an electron, or dot, from another element, this makes a bond, which is represented by a line (Fig. 2.29 C).
Fig. 2.29. (A) To make water, oxygen needs two additional electrons, which it can share with two hydrogens, which each need only one additional electron. (B) Single electrons match up to make pairs. (C) Lines between the paired electrons represent bonds.
Image by Byron Inouye
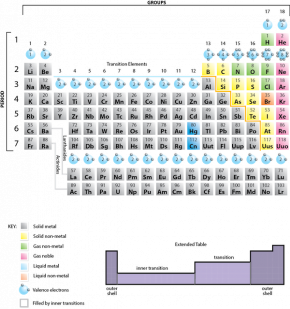
The number of bonds that an element can form is determined by the number of electrons in its valence shell (Fig. 2.29.1). Similarly, the number of electrons in the valence shell also determines ion formation. The octet rule applies for covalent bonding, with a total of eight electrons the most desirable number of unshared or shared electrons in the outer valence shell. For example, carbon has an atomic number of six, with two electrons in shell 1 and four electrons in shell 2, its valence shell (see Fig. 2.29.1). This means that carbon needs four electrons to achieve an octet. Carbon is represented with four unpaired electrons (see Fig. 2.29.1). If carbon can share four electrons with other atoms, its valence shell will be full.
Fig. 2.29.1. A periodic table showing electrons in the valence shells
Image by Byron Inouye
Most elements involved in covalent bonding need eight electrons to have a complete valence shell. One notable exception is hydrogen (H). Hydrogen can be considered to be in Group 1 or Group 17 because it has properties similar to both groups. Hydrogen can participate in both ionic and covalent bonding. When participating in covalent bonding, hydrogen only needs two electrons to have a full valence shell. As it has only one electron to start with, it can only make one bond.
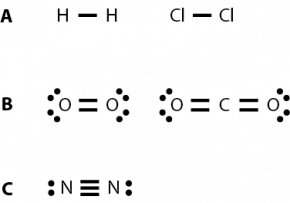
Fig. 2.30. Examples of single, double, and triple bonds (A) single bonds, H2 and Cl2 (B) double bonds, O2 and CO2 (C) triple bond, N2
Image by Byron Inouye
Single Bonds
Hydrogen is shown in Fig 2.28 with one electron. In the formation of a covalent hydrogen molecule, therefore, each hydrogen atom forms a single bond, producing a molecule with the formula H2. A single bond is defined as one covalent bond, or two shared electrons, between two atoms. A molecule can have multiple single bonds. For example, water, H2O, has two single bonds, one between each hydrogen atom and the oxygen atom (Fig. 2.29). Figure 2.30 A has additional examples of single bonds.
Double Bonds
Sometimes two covalent bonds are formed between two atoms by each atom sharing two electrons, for a total of four shared electrons. For example, in the formation of the oxygen molecule, each atom of oxygen forms two bonds to the other oxygen atom, producing the molecule O2. Similarly, in carbon dioxide (CO2), two double bonds are formed between the carbon and each of the two oxygen atoms (Fig. 2.30 B).
Triple Bonds
In some cases, three covalent bonds can be formed between two atoms. The most common gas in the atmosphere, nitrogen, is made of two nitrogen atoms bonded by a triple bond. Each nitrogen atom is able to share three electrons for a total of six shared electrons in the N2 molecule (Fig. 2.30 C).
Polyatomic Ions
In addition to elemental ions, there are polyatomic ions. Polyatomic ions are ions that are made up of two or more atoms held together by covalent bonds. Polyatomic ions can join with other polyatomic ions or elemental ions to form ionic compounds.
It is not easy to predict the name or charge of a polyatomic ion by looking at the formula. Polyatomic ions found in seawater are given in Table 2.10. Polyatomic ions bond with other ions in the same way that elemental ions bond, with electrostatic forces caused by oppositely charged ions holding the ions together in an ionic compound bond. Charges must still be balanced.
| Polyatomic Ion | Ion Name |
|---|---|
| NH4+ | ammonium |
| CO32- | carbonate |
| HCO3- | bicarbonate |
| NO2- | nitrite |
| NO3- | nitrate |
| OH- | hydroxide |
| PO43- | phosphate |
| HPO42- | hydrogen phosphate |
| SiO32- | silicate |
| SO32- | sulfite |
| SO42- | sulfate |
| HSO3- | bisulfite |
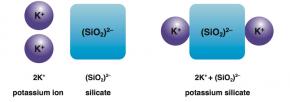
Fig. 2.31 shows how ionic compounds form from elemental ions and polyatomic ions. For example, in Fig. 2.31 A, it takes two K+ ions to balance the charge of one (SiO2)2- ion to form potassium silicate. In Figure 2.31 B, ammonium and nitrate ions have equal and opposite charges, so it takes one of each to form ammonium nitrate.
Fig. 2.31. (A) The formation potassium silicate
Image by Byron Inouye
Fig. 2.31. (B) The formation of ammonium nitrate
Image by Byron Inouye
Polyatomic ions can bond with monatomic ions or with other polyatomic ions to form compounds. In order to form neutral compounds, the total charges must be balanced.
Comparison of Ionic and Covalent Bonds
A molecule or compound is made when two or more atoms form a chemical bond that links them together. As we have seen, there are two types of bonds: ionic bonds and covalent bonds. In an ionic bond, the atoms are bound together by the electrostatic forces in the attraction between ions of opposite charge. Ionic bonds usually occur between metal and nonmetal ions. For example, sodium (Na), a metal, and chloride (Cl), a nonmetal, form an ionic bond to make NaCl. In a covalent bond, the atoms bond by sharing electrons. Covalent bonds usually occur between nonmetals. For example, in water (H2O) each hydrogen (H) and oxygen (O) share a pair of electrons to make a molecule of two hydrogen atoms single bonded to a single oxygen atom.
In general, ionic bonds occur between elements that are far apart on the periodic table. Covalent bonds occur between elements that are close together on the periodic table. Ionic compounds tend to be brittle in their solid form and have very high melting temperatures. Covalent compounds tend to be soft, and have relatively low melting and boiling points. Water, a liquid composed of covalently bonded molecules, can also be used as a test substance for other ionic and covalently compounds. Ionic compounds tend to dissolve in water (e.g., sodium chloride, NaCl); covalent compounds sometimes dissolve well in water (e.g., hydrogen chloride, HCl), and sometimes do not (e.g., butane, C4H10). Properties of ionic and covalent compounds are listed in Table 2.11.
| Property | Ionic | Covalent |
|---|---|---|
| How bond is made | Transfer of e- | Sharing of e- |
| Bond is between | Metals and nonmetals | Nonmetals |
| Position on periodic table | Opposite sides | Close together |
| Dissolve in water? | Yes | Varies |
| Consistency | Brittle | Soft |
| Melting temperature | High | Low |
The properties listed in Table 2.11 are exemplified by sodium chloride (NaCl) and chlorine gas (Cl2). Like other ionic compounds, sodium chloride (Fig. 2.32 A) contains a metal ion (sodium) and a nonmetal ion (chloride), is brittle, and has a high melting temperature. Chlorine gas (Fig. 2.32 B) is similar to other covalent compounds in that it is a nonmetal and has a very low melting temperature.
Fig. 2.32. (A) sodium chloride (NaCl), an ionic compound
Image courtesy of Edal Anton Lefterov from Wikipedia
Fig. 2.32. (B) chlorine gas (Cl2), a covalent compound
Image courtesy of Tomihahndorf from Wikipedia
Dissolving, Dissociating, and Diffusing
Ionic and covalent compounds also differ in what happens when they are placed in water, a common solvent. For example, when a crystal of sodium chloride is put into water, it may seem as though the crystal simply disappears. Three things are actually happening.
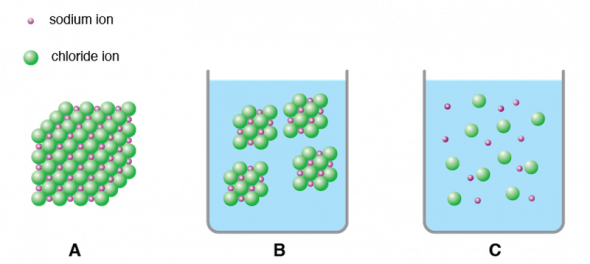
- A large crystal (Fig. 2.33 A) will dissolve, or break down into smaller and smaller pieces, until the pieces are too small to see (Fig. 2.33 B).
- At the same time, the ionic solid dissociates, or separates into its charged ions (Fig 2.33 C).
- Finally, the dissociated ions diffuse, or mix, throughout the water (Fig 2.34).
Fig 2.33. Dissolution and dissociation of sodium chloride. Sodium and chloride ions in (A) a large crystal, (B) dissolved in water as smaller crystals, and (C) dissociated in water.
Image by Byron Inouye
Ionic compounds like sodium chloride dissolve, dissociate, and diffuse. Covalent compounds, like sugar and food coloring, can dissolve and diffuse, but they do not dissociate. Fig. 2.34, is a time series of drops of food coloring diffusing in water. Without stirring, the food coloring will mix into the water through only the movement of the water and food coloring molecules.
Fig. 2.34. A time series of food coloring diffusing in water
Image by Jordan Wang
Dissociated sodium (Na+) and chloride (Cl-) ions in salt solutions can form new salt crystals (NaCl) as they become more concentrated in the solution. As water evaporates, the salt solution becomes more and more concentrated. Eventually, there is not enough water left to keep the sodium and chloride ions from interacting and joining together, so salt crystals form. This occurs naturally in places like salt evaporation ponds (Fig. 2.35 A), in coastal tidepools, or in hot landlocked areas (Fig. 2.35 B). Salt crystals can also be formed by evaporating seawater in a shallow dish, as in the Recovering Salts from Seawater Activity.
Fig. 2.35. (A) Salt evaporation ponds on the shores of San Francisco Bay
Image courtesy of Doc Searls
Fig. 2.35. (B) Salt crystals in Badwater Basin, the lowest point in North America in Death Valley National Park
Image courtesy of Arlene Philippoff