Matter in the Sea
Clarification Statement: Examples of evidence could include adding air to expand a basketball, compressing air in a syringe, dissolving sugar in water, and evaporating salt water.
Assessment Boundary: Assessment does not include the atomic-scale mechanism of evaporation and condensation or defining unseen particles.
What is Matter?
Fig. 1. Everything in this cityscape of Honolulu, Hawai'i is made of matter!
Image courtesy of Wikimedia
Matter is everything around you. Matter takes up space and has mass. Even when matter is too small to see, it can be detected by weighing with very sensitive scales or by studying the matter's effects on other objects.
Matter is conserved—the amount stays the same—even when it changes form, or is broken down into parts too small to see. For example, water can change from a solid to a liquid, or to a gas, but in each form, the amount of matter stays the same.
Matter is described by the types of atoms it contains. The state of matter is described and predicted by the types, interactions, and motions of the atoms within it (Fig. 2).
Fig. 2. Matter in all states is made up of particles too small to be seen.
Image courtesy of PlusPNG adapted by Emily Sesno
Matter in the Sea
Fig. 3. The view of Hawaii from space shows the abundance of water on Earth.
Image captured by Google Earth
The ocean covers 70% of Earth's surface (Fig. 3). Seawater is a dynamic chemical mixture that has constant input of matter from the land, atmosphere, and living things. Sometimes we can see some of the materials in the ocean. For example, when rainwater flows over the land, it dissolves substances from soil and rocks. Runoff carries these materials directly into the ocean or into streams and rivers that empty into the ocean (Fig. 4).
Fig. 4. An aerial image of the Gulf of Mexico shows runoff and resuspended sediments in the hours after Tropical Storm Ida in 2009.
Image courtesy of NASA Earth Observatory
However, most particles in the ocean are invisible to the naked eye. Rain falling into the ocean carries gases and small particles of soot and dust. Atmospheric gases mix and dissolve into seawater, especially when winds and waves churn the ocean surface. Seawater also dissolves materials from the ocean bottom, as well as materials released by underwater volcanoes and hydrothermal vents. The constant addition of dissolved substances into the ocean over billions of years has made the ocean salty.
Salinity
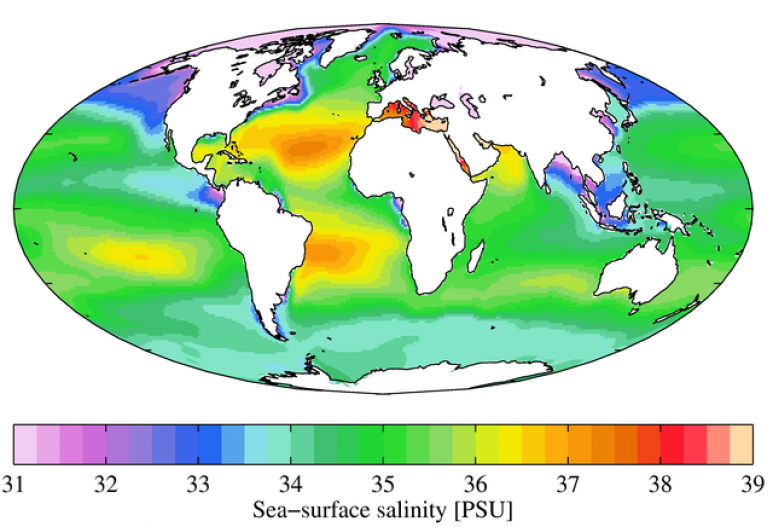
Salt is a mineral made up of sodium (Na) and chloride (Cl), forming the compound NaCl. We often refer to the amount of salt (or salinity) in solution as the amount of salt in 1,000 g of water, or 'parts per thousand' (ppt). The ocean has an average salinity about 35 ppt, which is mostly NaCl but also includes other salts in addition to NaCl such as magnesium. The salinity around the globe changes depending on other factors such as freshwater input or runoff from land (Fig. 5). Input from various sources increases the amount of dissolved matter in the ocean and can influence the salinity.
Fig. 5. Salinity in the ocean varies by area, ranging from 31 to 39 ppt, but the average is 35 ppt. Data shown here represent the sea surface salinity in 2009 and are from the World Ocean Atlas. The units shown here, Practical Salinity Units (PSU), represent the same information as Parts Per Thousand (ppt).
Image courtesy Wikimedia Commons
Fig. 6. Water is a very good solvent that easily dissolves many solutes, like salt, forming a solution.
Image adapted from Wikimedia Commons by Emily Sesno
About 97% of all the water on Earth is salty, or saline. In fact, many lakes are salty! You can't see saltiness just by looking because salts are dissolved. Most of the water on earth, including in oceans, lakes, rivers, and ponds, contains many different solutes. In the solution of seawater, water is the solvent, salt is the solute, and the resulting saltwater is the solution (Fig. 6). Water is a very good solvent.
Salt is a common substance that we are familiar with in many forms, such as table salt, rock salt, and sea salt. When seawater evaporates, salt is left behind. If seawater evaporates from a surface with a slight curve, such as a watch glass or a shallow tidepool, the puddle of water will get progressively smaller. Different types of salts will settle out depending on their solubility, causing distinct rings (Fig. 7). Each ring is made up of different types of salts, from the original seawater mixture.
Fig. 7. A close-up of salt rings on watch glass.
Image by Joanna Philippoff
Salt Harvesting
Traditionally, salt was harvested from either solar evaporation ponds or rock deposits. Salt evaporation ponds are shallow, artificial basins designed to extract salt from salty water through natural evaporation. As the water dries up, salt crystals are harvested by raking. Salt evaporation ponds are almost entirely located in warm climates, where there is high evaporation and low precipitation (little rain). Today, seawater is sometimes filtered to remove impurities before solar evaporation. To learn more about salt harvesting in Hawai'i, click on the Traditional Ways of Knowing special feature below.