Research
Published in Trends in Biochemical science (TiBs):
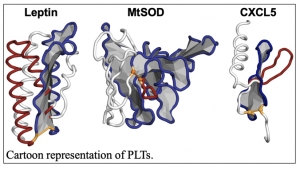
“Topological Twists in Nature,” by Dr. Ellinor Haglund and Ms. Jennifer Simien provides insights on a recently discovered protein structure that influences human health. See the news release.
Latest Important Information
Registration Information is available!
Need a course override? See our FAQ and our Override Request Form. Students from outside UH, please follow the instructions that accompany the form for submitting your transcript with your override request.
Registration questions? Please see our FAQ here.
CHEM 131/161/171 Placement Exams – Click for testing dates.
Are you an incoming Freshman majoring in Chemistry? If you would like an advising session, please schedule your appointment online!
Still have registration questions? chemreg@hawaii.edu